The synthesized vinyl ethyl ether b p.
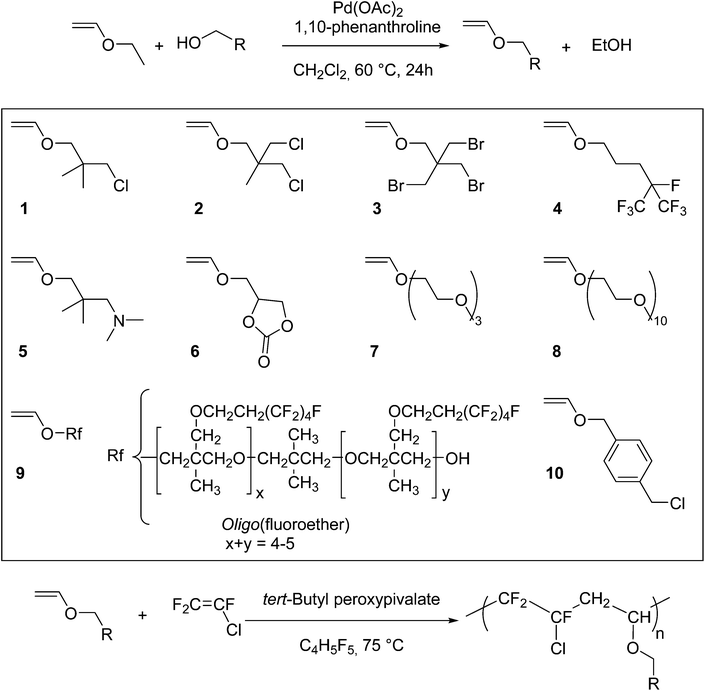
Ethyl vinyl ether reaction with alcohol.
35 5 deg is accompanied by diethyl ether b p.
Proton magnetic resonance analysis of the reaction products shows that.
The first two reactions proceed by a sequence of s n 2 steps in which the iodide or bromide anion displaces an alcohol in the first step and then converts the conjugate acid of that alcohol to an alkyl halide in the second.
To a solution of the alcohol 1 0 equiv in ethyl vinyl ether 10 equiv was added hg oac 2 0 1 equiv.
Ethyl vinyl ether participates in many reactions of interest to organic synthesis.
Google has not performed a legal analysis and makes no representation as to the.
In formic acid and acetic acid buffer solutions it shows general acid catalysis.
Abstract two novel trifluorovinyl ether tfve monomers were copolymerized with either ethyl vinyl ether eve or vinyl acetate vac in a redox initiated aqueous emulsion.
Ethyl vinyl ether stabilized with koh 純度 試験方法.
The conjugate acid of the ether is an intermediate in all these reactions just as conjugate acids were intermediates in certain alcohol reactions.
The reaction is first order in ethyl vinyl ether and first order in hydronium ion.
With catalytic amounts of acids ethyl vinyl ether adds to alcohols to give the mixed acetal.
The reaction mixture was stirred at reflux under n 2 for 24 h then the excess ethyl vinyl ether was removed under vacuum.
Handling storage and precautions.
Ethyl vinyl ether and n butyl vinyl ether may be distilled from k 2 co 3 or na if purification of the commercial materials is desirable.
34 6 deg and other impurities.
A satisfactory separation has been made by gas chromatography in a 2 m dimethylsulfolane column followed by a 3 m squalane column on celite.
Etoch ch 2 roh etoch or ch 3 this alcohol protection reaction is akin to the behavior of dihydropyran.